The purpose of establishment
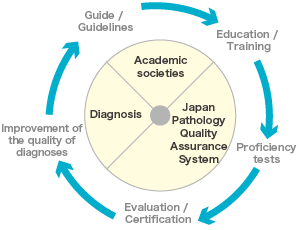
In Japan, pathological diagnosis departments are required by Cabinet Order. They contribute significantly to improving the quality of medical services by providing definite diagnoses of diseases based on cell and tissue morphology. To provide quality pathological diagnoses at every medical institution in Japan, it is crucial to standardize not only the pathological techniques and diagnostic criteria employed, but also the quality assurance system within and outside pathological diagnosis departments.
As part of the quality assurance system for pathological diagnosis, individual institutions are responsible for internal checks on accuracy, such as the staining required for specimen preparation and microscopic observation, and the diagnosis and reporting processes used by pathologists at medical institutions. External evaluation of accuracy has been conducted only at the study level, mainly by the Japanese Society of Pathology. Thus far, although differences in diagnostic accuracy between institutions have been observed, no systematic programs have been put in place to ensure improved accuracy.
To face these challenges and provide clinicians with appropriate and high-quality pathological diagnoses, as well as to fulfill our responsibilities to patients and citizens, there is a need to establish an organization dedicated to the assurance of accurate pathological diagnoses. Establishing an organization engaged in providing an accuracy assurance system will increase the public’s awareness of the need for such a system. We plan to deploy programs to regularly evaluate the quality of pathological diagnosis in multiple institutions to determine what areas require improvement and to enhance their improvement strategies. The continuous implementation of accuracy assurance programs such as this will allow pathologists to take responsibility for their pathological diagnoses, clinicians to provide medical treatment with confidence based on such pathological diagnosis, and citizens to benefit from accurate pathological diagnoses and medical treatment. Furthermore, the more efficient use of medical funding will improve the quality of medical services provided to citizens.
The activities of our organization are mainly carried out by pathologists and medical technologists who are directly involved in pathological diagnosis. However, we aim to provide programs that are open to the public, in cooperation with clinical societies and patient groups who have a strong interest in what we are doing.
Some other countries have a history of conducting external evaluations of this type to ensure accuracy and have achieved successful results that vitally support their medical services. In Japan, quality improvement of pathological diagnoses has thus far been promoted chiefly by the Japan Society of Pathology. However, given the need for a consistent accuracy assurance system and the public nature of pathological diagnosis in medical care services, we have established a non-profit organization (NPO), aiming at contributing to the improvement of medical services for citizens.
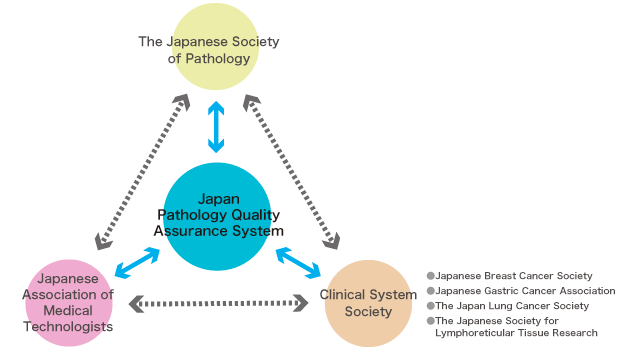
The positioning of our organization in medical care services and related academic societies
The Japan Pathology Quality Assurance System (an NPO) implements accuracy management in cooperation with academic societies and other organizations. Our aim is to standardize pathology techniques and diagnostic criteria, to improve the quality of pathological diagnoses.
We have established a system to provide organic and efficient services. The regular members of our organization include the following academic societies.
Organization
Members of the board of directors
- President
- Hiroshi KIJIMA
- Vice presidents
- Shinobu MASUDA
- Hironori KATAYAMA
- Auditors
- Ichiro MAEDA
- Hitoshi ABE
- Directors
- Atsushi OCHIAI
- Reiji HABA
- Shuichiro FURUYA
- Eiichi MORII
- Atsushi KISANUKI
- Yuko SASAJIMA
- Masayuki YOSHIDA
- Takashi SAKATANI
- Takeshi KUWATA
- Yutaka HATANAKA
- Takeshi SASAKI
- Kouji TSUTA
- Junichiro IKEDA
- Executive director
- Hisashi TAKINO
- Kyoko KOMATSU
Members of the committee
1. Project management committee
- Chairman
- Hiroshi KIJIMA
- Committee member
- Shinobu MASUDA
- Hironori KATAYAMA
- Eiichi MORII
- Takeshi SASAKI
- Atsushi ASANO
2. Negotiation committee
- Chairman
- Atsushi OCHIAI
3. Public relations committee
- Chairman
- Yuko SASAJIMA
- Committee member
- Yutaka HATANAKA
- Yuuji Aoki
4. Education & workshop committee
- Chairman
- Reiji HABA
- Committee member
- Atsushi KISANUKI
- Akira KUROSE
- Kyoko KOMATSU
- Hitoshi ABE
5. Evaluation committee
- Chairman
- Shinobu MASUDA
- Vice Chairman
- Takashi SAKATANI
- Committee member
- Hiroshi KIJIMA
- Takeshi KUWATA
- Masayuki YOSHIDA
- Kyoko KOMATSU
- Shuichiro FURUYA
- Hironori KATAYAMA
- Hisashi TAKINO
6. Ethics committee
- Chairman
- Yutaka HATANAKA
- Committee member
- Takeshi KUWATA
- Eiichi MORII
- Takeshi SASAKI
- Mika WATANABE
- Hisashi TAKINO
- Masako KOTANI
7. Internationalization committee
- Committee member
- Kanako HATANAKA
8. Evaluation subcommittee
- Chairman
- Reiji HABA
- Committee member
- Shinobu MASUDA
- Yutaka HATANAKA
- Atsushi KISANUKI
- Takeshi KUWATA
- Hiroshi KIJIMA
- Kazuki NABESHIMA
- Yoko NAKANISHI
- Chiho OBAYASHI
- Committee member
- Yuko SASAJIMA
- Hisashi TAKINO
- Hitoshi TSUDA
- Ryo WADA
- Masayuki YOSHIDA
- Aya USHIKU
- Junichiro IKEDA
- Kenichi KOHASHI
- Kanako HATANAKA
- Kazuya YAMASHITA
- Shuhei ISHII
- Committee member
- Yuuji Aoki
- Shinji HAMAKAWA
- Atsushi ASANO
- Hitoshi ABE





